
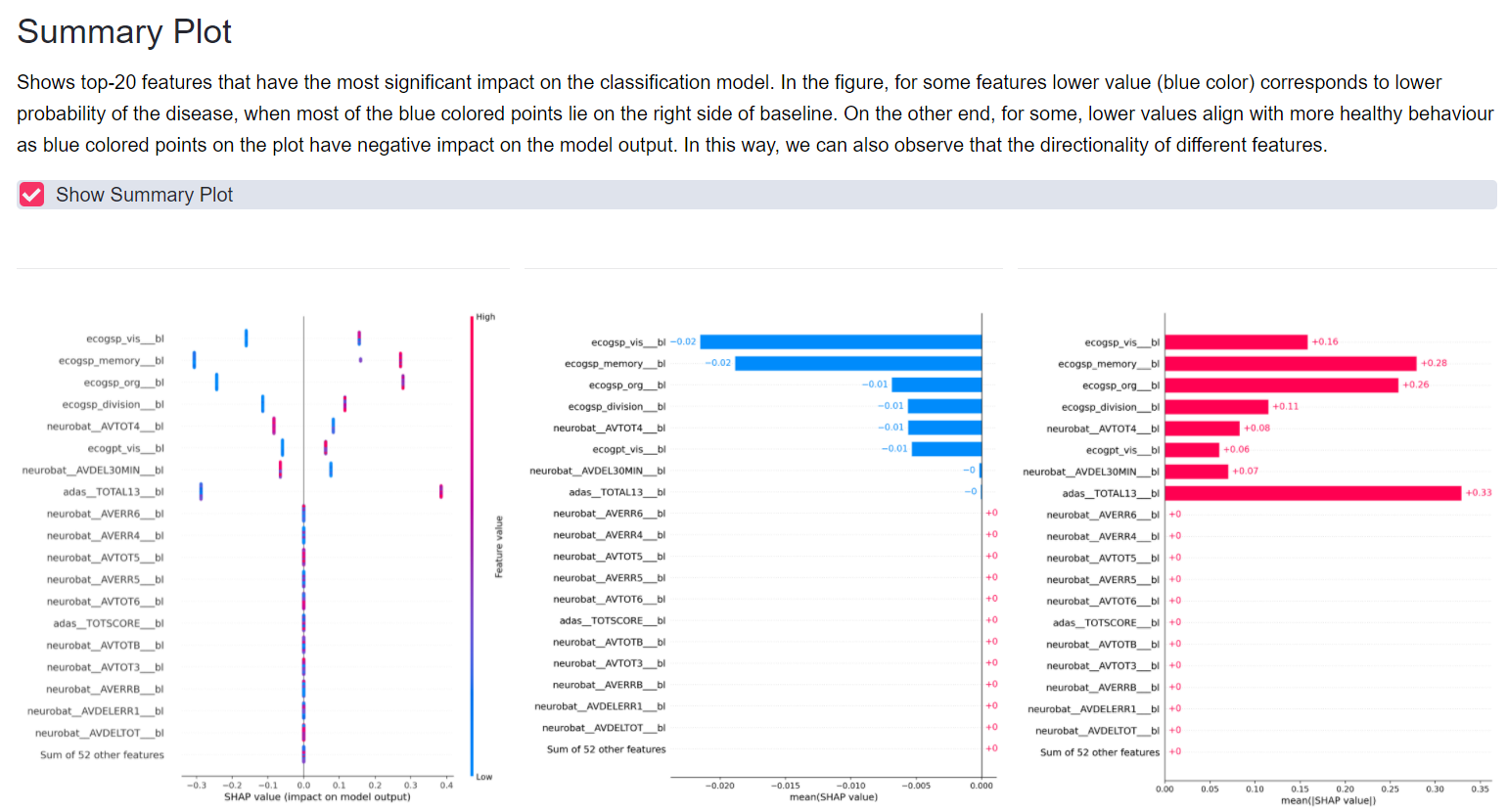
Figure: Dementia progression subtyping and longer term disease trajectory prediction via machine learning.
| Vipul Satone, Rachneet Kaur, Anant Dadu, Hampton Leonard, Hirotaka Iwaki, Mary Makarious, Lana Sargent, Ali Daneshmand, Sonja W. Scholz, Mike A. Nalls, Roy H. Campbell, Faraz Faghri |
|
University of Illinois at Urbana-Champaign, The National Institutes of Health Under Submission, 2020 |
|
|
|
|
|
Abstract |
|
Background: Alzheimer’s disease (AD) is a common, age-related, neurodegenerative disease that impairs a person’s ability to perform day-to-day activities. Diagnosing AD is challenging, especially in the early stages. Many patients still go undiagnosed, partly due to the complex heterogeneity in disease progression. This highlights a need for early prediction of the disease course to assist its treatment and tailor therapy options to the disease progression rate. Recent developments in machine learning techniques provide the potential to not only predict disease progression and trajectory of AD but also to classify the disease into different etiological subtypes.
Methods and findings: The work shown here clusters participants in distinct and multifaceted progression subgroups of AD and discusses an approach to predict the progression rate from baseline diagnosis. We observed that the myriad of clinically reported symptoms summarized in the proposed AD progression space corresponds directly with memory and cognitive measures, which are routinely used to monitor disease onset and progression. Our analysis demonstrated accurate prediction of disease progression after four years from the first 12 months of post-diagnosis clinical data (Area Under the Curve of 0.96 (95% confidence interval (CI), 0.92-1.0), 0.81 (95% CI, 0.74-0.88) and 0.98 (95% CI, 0.96-1.0) for slow, moderate and fast progression rate patients respectively). Further, we explored the long short-term memory (LSTM) neural networks to predict the trajectory of an individual patient’s progression. Conclusion: The machine learning techniques presented in this study may assist providers in identifying different progression rates and trajectories in the early stages of the disease, hence allowing for more efficient and personalized healthcare deliveries. With additional information about the progression rate of AD at hand, providers may further individualize the treatment plans. The predictive tests discussed in this study not only allow for early AD diagnosis but also facilitate the characterization of distinct AD subtypes relating to trajectories of disease progression. These findings are a crucial step forward for early disease detection. These models can be used to design improved clinical trials for AD research. |

Figure: Dementia progression subtyping and longer term disease trajectory prediction via machine learning. |
Interactive web portal |
|
References |
| Vipul K Satone, Rachneet Kaur, Anant Dadu, Hampton Leonard, Hirotaka Iwaki, Mary Makarious, Lana Sargent, Ali Daneshmand, Sonja W Scholz, Andrew B Singleton, Mike A Nalls, Roy H Campbell, Faraz Faghri, Alzheimer's Disease Neuroimaging Initiative. Predicting Alzheimer’s disease progression trajectory and clinical subtypes using machine learning. In bioRxiv (2020): 792432 [BibTex] [Text] |

|
Acknowledgements |
| This work was supported in part by the Intramural Research Programs of the National Institute on Aging and the National Institute of Neurological Disorders and Stroke (project numbers: Z01-AG000949-02 and ZIA-NS003154). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. RK is thankful to William Chittenden for the William A. Chittenden Award. |
| Website adapted from Jingxiang, Richard and Deepak. |